What is the chemical name of SI6?
Silylpentasilolane
| PubChem CID | 13908658 |
|---|---|
| Structure | Find Similar Structures |
| Molecular Formula | Si6 |
| Synonyms | Silylpentasilolane DTXSID50761058 101753-14-4 |
| Molecular Weight | 168.51 |
How many valence electrons are in SI6?
SI6 ‘s molecular geometry is octahedral. Let’s start with its Lewis structure. S has 6 valence electrons and each I atom has 7 valence electrons, for a total of 6+6⋅7=48 electrons that need to be accounted for.
What is the name of BH2?
Boron dihydride
| PubChem CID | 139760 |
|---|---|
| Molecular Formula | BH2 |
| Synonyms | Boron dihydride dihydridoboron(.) BH2 Radical 14452-64-3 Borane(2) More… |
| Molecular Weight | 12.83 |
| Dates | Modify 2022-05-28 Create 2004-09-16 |
What element is SI3?
SI3 : Summary
| Code | SI3 |
|---|---|
| One-letter code | X |
| Molecule name | 5-(acetylamino)-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid |
| Synonyms | N-acetylneuraminic acid, ketone form |
What is the name of BeF2?
Beryllium fluoride
| PubChem CID | 24589 |
|---|---|
| Molecular Formula | BeF2 |
| Synonyms | BERYLLIUM FLUORIDE Beryllium difluoride 7787-49-7 Beryllium fluoride (BeF2) beryllium;difluoride More… |
| Molecular Weight | 47.008989 |
| Component Compounds | CID 14917 (Hydrofluoric acid) CID 5460467 (Beryllium) |
What is h2 S o4 called?
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula H2SO4.
Is si6 polar or non polar?
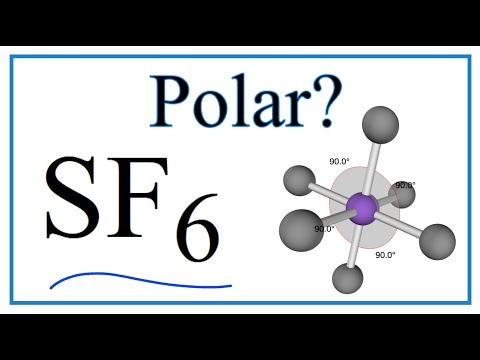
How many lone pairs are in SCl6?
Lewis structure of SCl6 contains six single bonds between the Sulfur (S) atom and each Chlorine (Cl) atom. The Sulfur atom (S) is at the center and it is surrounded by 6 Chlorine atoms (Cl). The Sulfur atom does not have a lone pair while all the 6 Chlorine atoms have 3 lone pairs.
What is ibr2?
University Professor – General and Organic Chemistry. Iodine dibromide.
What is the the shape molecular geometry of BH2 -?
Re: Shape of BH2- [ENDORSED] The lone pair on boron will repel the two B-H bonds away from it. Thus, the shape is actually bent and not linear. Furthermore, the angles are not exactly 120 degrees as in the case of a trigonal planar molecule where the surrounding atoms are all the same.
What is borane made of?
borane, any of a homologous series of inorganic compounds of boron and hydrogen or their derivatives.
How is BeH2 formed?
What is po43?
PO43- is a chemical derivative of phosphoric acid with a chemical name Phosphate. Phosphate is also called Phosphate ion or Orthophosphate. It is a trivalent inorganic anion and a conjugate base of hydrogen phosphate.
How many atoms are in Silicon phosphate?
3.1Computed Properties
| Property Name | Property Value | Reference |
|---|---|---|
| Heavy Atom Count | 23 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 36.8 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
What is the formula of silicon?
Silicon
| PubChem CID | 5461123 |
|---|---|
| Chemical Safety | Laboratory Chemical Safety Summary (LCSS) Datasheet |
| Molecular Formula | Si |
| Synonyms | 7440-21-3 Si Silicon Silicone Silicon metal More… |
| Molecular Weight | 28.085 |
Does BeF2 exist?
Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.
How do you draw BeF2?
How is BeF2 formed?
Formation of BeF2: (iii) The half filled 2pz orbitals of two fluorine atoms overlap with ‘sp’ hybrid orbitals of beryllium in their axis to form two (sp – p) bonds. (iv) BeF2 molecule so formed has linear shape.
